
COMMITTED TO OBSTETRICS CARE
For over 50 years, Ferring has been dedicated to the development of treatments for obstetric care.
This section has been designed to offer health care professionals a quick, user-friendly way to find clinical information on CERVIDIL, get detailed step-by-step instructions on CERVIDIL use, determine the appropriate patient for cervical ripening, and access the current clinical evidence.
HELP RIPEN THE CERVIX WITH CERVIDIL AT YOUR FINGERTIPS
- Each insert contains 10 mg dinoprostone (PGE2) dispersed throughout its matrix, and releases approx. 0.3 mg/hour PGE2 over a 12-hour period to maintain constant release.1**
- CERVIDIL is used as a single dose in a single application to initiate or continue cervical ripening1
- CERVIDIL’s retrieval system ensures easy and reliable removal of the insert1
-
-
** Clinical significance has not been established
-
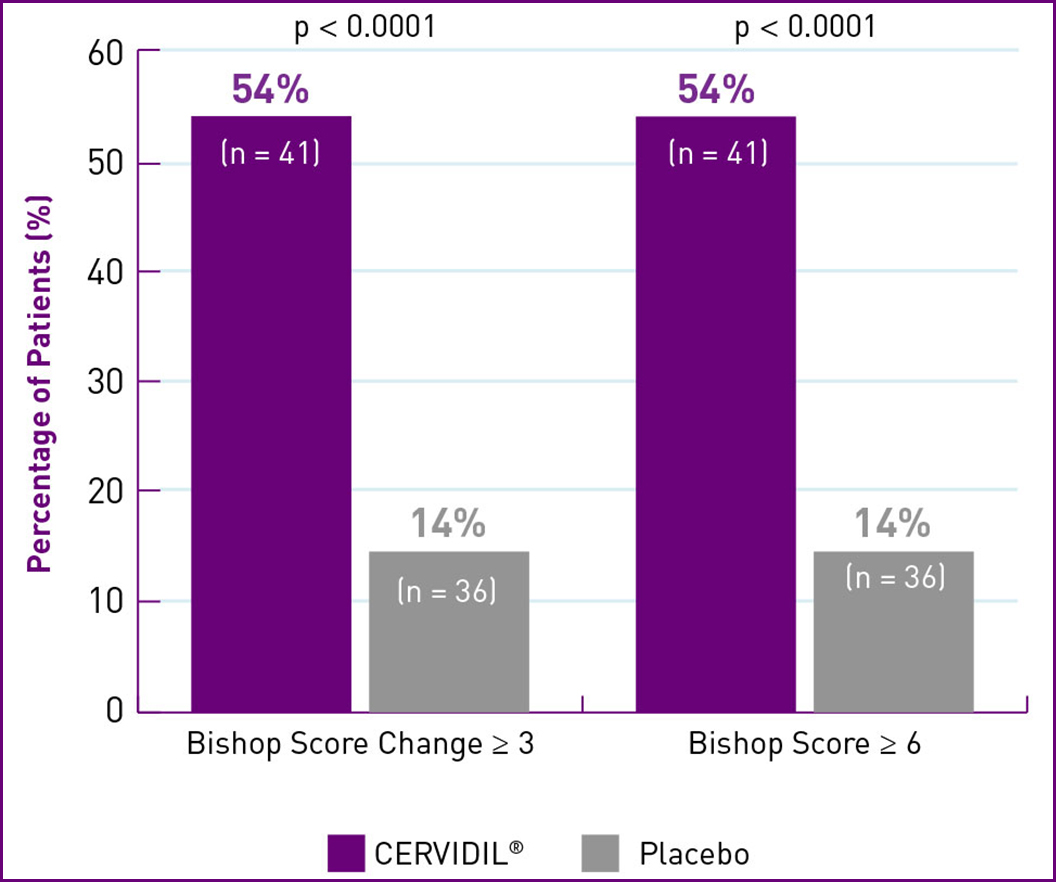
CERVIDIL SHOWED STATISTICALLY SIGNIFICANT CERVICAL RIPENING AFTER 12 HOURS1,2*†‡§
NULLIPARAS/PRIMIPARAS
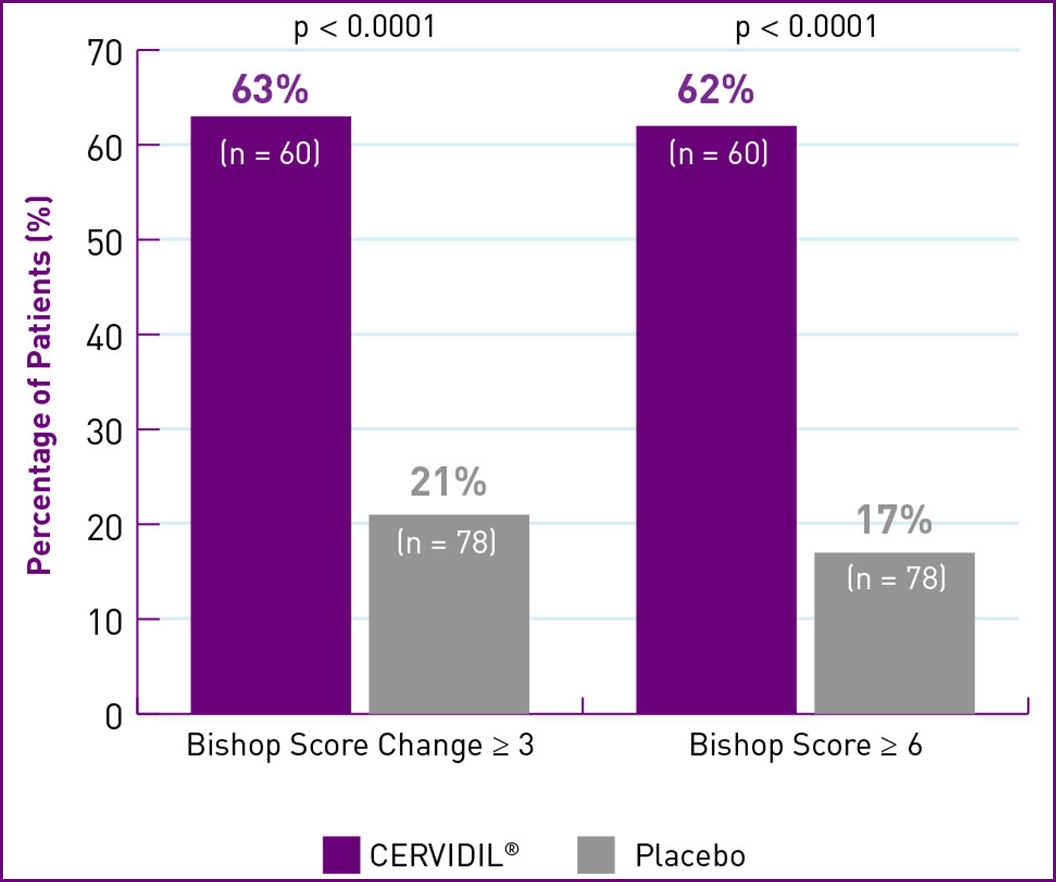
Adapted from Rayburn WF, et al. 2
MULTIPARAS
| * | A randomized, double-blind study of 215 patients scheduled to undergo labour induction designed to evaluate the efficacy and safety of CERVIDIL. All patients had an entry Bishop score of 4 or less and gestational age of 37 or more weeks. During the 12-hour observation period, clinical endpoints included changes in Bishop cervical score and onset of labour. |
| † | Median Bishop score at baseline: Nulliparas patients – CERVIDIL=2.0; Placebo=2.0. Patients with score change ≥ 3: CERVIDIL group: 63% (n=60);Placebo group: 21% (n=78). With score ≥ 6: CERVIDIL group: 62% (n=60); Placebo group: 17% (n=78). Multiparas patients – CERVIDIL=3.0 ; Placebo=3.0. Patients with score change ≥ 3: CERVIDIL group: 54% (n=41); Placebo group: 14% (n=36). With score ≥ 6: CERVIDIL group: 54% (n=41); Placebo group: 14% (n=36). (p<0.0001 for all comparison groups). |
| ‡ | Measurable effect was defined as an increase in Bishop score of 3 or more points above baseline or an absolute score of 6 or higher. |
| § | Treatment success was considered a change in Bishop score after 12 hours (treatment failure was defined as no change in Bishop score after 12 hours). |
IN A CLINICAL TRIAL, ALL CASES OF HYPERSTIMULATION REVERSED WITHIN 2 TO 13 MINUTES OF REMOVING CERVIDIL.1
ESTABLISHED SAFETY PROFILE
CERVIDIL IS GENERALLY WELL-TOLERATED1
- Drug-related fever, nausea, vomiting, diarrhea, and abdominal pain were noted in < 1% of CERVIDIL patients
- Controlled study with retrieval system. Total related adverse events vs. placebo (active group – n=102, placebo – n=104: uterine hyperstimulation with fetal distress: 2.9% vs. 0%; uterine hyperstimulation without fetal distress: 2.0% vs. 0%; fetal distress without uterine hyperstimulation: 2.9% vs. 1%)
CERVIDIL SAFETY INFORMATION
Indication and clinical use:
CERVIDIL (dinoprostone) is indicated for: Initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetrical indication for the induction of labour. CERVIDIL is not recommended in the geriatric and pediatric populations.
Contraindications:
- Patients in whom there is clinical suspicion or definite evidence of fetal distress where delivery is not imminent
- Patients with placenta previa or unexplained vaginal bleeding during this pregnancy
- Patients in whom there is evidence or strong suspicion of marked cephalopelvic disproportion
- Patients in whom oxytocic drugs are contraindicated or when prolonged contraction of the uterus may be detrimental to fetal safety or uterine integrity (previous caesarean section or major uterine surgery)
- Multipara with 6 or more previous term pregnancies
- Patients with a history of difficult labour and/or traumatic delivery
- Patients with overdistension of uterus (multiple pregnancy, polyhydramnios)
- Patients with fetal malpresentation
- Patients with a history of epilepsy whose seizures are poorly controlled
- Should not be used simultaneously with other oxytocics
- Should not be used when there is a history of, or current pelvic inflammatory disease, unless adequate prior treatment has been instituted
Most serious warnings and precautions:
For Hospital Use Only: CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Other relevant warnings and precautions:
- Removal prior to oxytocin administration
- Removal if uterine hyperstimulation is encountered or if labour commences; prior to amniotomy; if there is fetal distress; if there is evidence of maternal or fetal adverse reactions
- Caution in patients with a history of previous uterine hypertonicity, glaucoma, or childhood asthma
- Caution in patients at risk for developing disseminated intravascular coagulation
- Evaluation of cephalopelvic relationships
- Caution in patients with severe renal disease and/or severe hepatic disease accompanied by metabolic aberrations
- Not indicated for use during early or other phases of pregnancy or during lactation
- Monitoring: After insertion, the patient should remain supine and monitored for 2 hours for any evidence of uterine hyperstimulation, change in fetal heart rate or maternal blood pressure or heart rate
For more information:
Please consult product monograph https://www.ferring.ca/media/1026/cervidil-approved-pm-english-september-2006.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
CERVIDIL - YOUR PESSARY FOR CERVICAL RIPENING1
- First and only dinoprostone vaginal insert for the induction of cervical ripening1*
- 12-hour controlled and constant release of dinoprostone**
- Active drug in hydrogel polymer matrix
- Rapid retrieval system ensures quick and reliable removal of the insert1‡
- Knitted polyester retrieval tape helps ease removal
-
* Comparative clinical significance has not been established ** Clinical significance has not been established ‡ Measurable effect was defined as an increase in Bishop score of 3 or more points above baseline or an absolute score of 6 or higher

PROPER ADMINISTRATION WILL HELP OPTIMIZE DRUG DELIVERY1†
- No sterile conditions are required for CERVIDIL insertion
- Patients should remain in the supine position for 2 hours following insertion

| 1. | Remove CERVIDIL from freezer and open the aluminum foil at the tear provided |
| 2. | Pull out the CERVIDIL vaginal delivery system using the retrieval tape |
| 3. | Position CERVIDIL insert securely between the middle and index fingers |
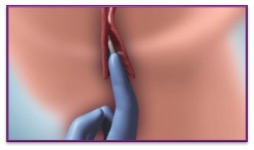
| 4. | Introduce CERVIDIL high up into the vagina assisted by a small amount of aqueous gel |
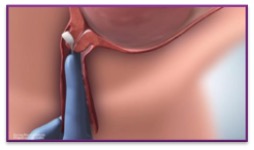
| 5. | Position CERVIDIL behind the posterior vaginal fornix transversely to ensure it remains in situ |
PROPER ADMINISTRATION
IS CRITICAL TO AVOID
POTENTIAL RISK
AND SHOULD BE
PERFORMED BY
TRAINED PERSONNEL
†Please refer to the Product Monograph for complete dosing and administration information
CERVIDIL SAFETY INFORMATION
Indication and clinical use:
CERVIDIL (dinoprostone) is indicated for: Initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetrical indication for the induction of labour. CERVIDIL is not recommended in the geriatric and pediatric populations.
Contraindications:
- Patients in whom there is clinical suspicion or definite evidence of fetal distress where delivery is not imminent
- Patients with placenta previa or unexplained vaginal bleeding during this pregnancy
- Patients in whom there is evidence or strong suspicion of marked cephalopelvic disproportion
- Patients in whom oxytocic drugs are contraindicated or when prolonged contraction of the uterus may be detrimental to fetal safety or uterine integrity (previous caesarean section or major uterine surgery)
- Multipara with 6 or more previous term pregnancies
- Patients with a history of difficult labour and/or traumatic delivery
- Patients with overdistension of uterus (multiple pregnancy, polyhydramnios)
- Patients with fetal malpresentation
- Patients with a history of epilepsy whose seizures are poorly controlled
- Should not be used simultaneously with other oxytocics
- Should not be used when there is a history of, or current pelvic inflammatory disease, unless adequate prior treatment has been instituted
Most serious warnings and precautions:
For Hospital Use Only: CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Other relevant warnings and precautions:
- Removal prior to oxytocin administration
- Removal if uterine hyperstimulation is encountered or if labour commences; prior to amniotomy; if there is fetal distress; if there is evidence of maternal or fetal adverse reactions
- Caution in patients with a history of previous uterine hypertonicity, glaucoma, or childhood asthma
- Caution in patients at risk for developing disseminated intravascular coagulation
- Evaluation of cephalopelvic relationships
- Caution in patients with severe renal disease and/or severe hepatic disease accompanied by metabolic aberrations
- Not indicated for use during early or other phases of pregnancy or during lactation
- Monitoring: After insertion, the patient should remain supine and monitored for 2 hours for any evidence of uterine hyperstimulation, change in fetal heart rate or maternal blood pressure or heart rate
For more information:
Please consult product monograph https://www.ferring.ca/media/1026/cervidil-approved-pm-english-september-2006.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
IDENTIFYING YOUR PATIENT
The following information may assist you with patient selection.
FACTORS SHOWN TO IMPACT INDUCTION OF LABOUR3
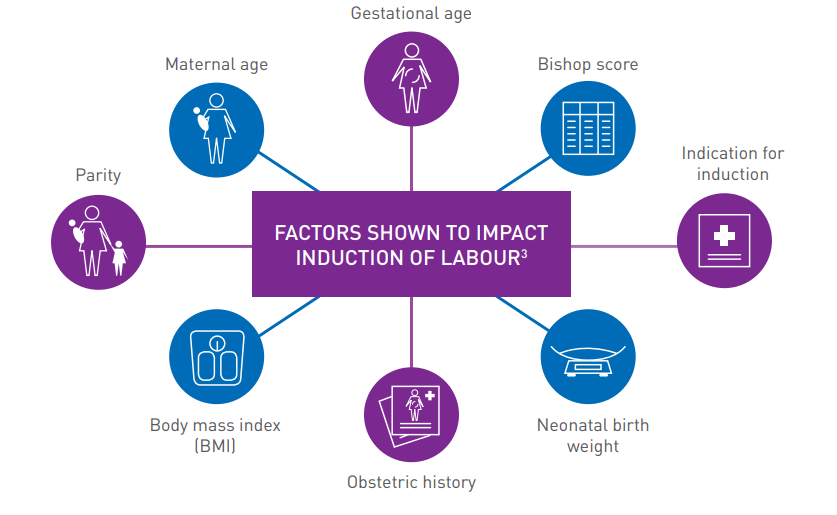
Treatment and care should take into account women’s individual needs and preferences.
CERVIDIL SAFETY INFORMATION
Indication and clinical use:
CERVIDIL (dinoprostone) is indicated for: Initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetrical indication for the induction of labour. CERVIDIL is not recommended in the geriatric and pediatric populations.
Contraindications:
- Patients in whom there is clinical suspicion or definite evidence of fetal distress where delivery is not imminent
- Patients with placenta previa or unexplained vaginal bleeding during this pregnancy
- Patients in whom there is evidence or strong suspicion of marked cephalopelvic disproportion
- Patients in whom oxytocic drugs are contraindicated or when prolonged contraction of the uterus may be detrimental to fetal safety or uterine integrity (previous caesarean section or major uterine surgery)
- Multipara with 6 or more previous term pregnancies
- Patients with a history of difficult labour and/or traumatic delivery
- Patients with overdistension of uterus (multiple pregnancy, polyhydramnios)
- Patients with fetal malpresentation
- Patients with a history of epilepsy whose seizures are poorly controlled
- Should not be used simultaneously with other oxytocics
- Should not be used when there is a history of, or current pelvic inflammatory disease, unless adequate prior treatment has been instituted
Most serious warnings and precautions:
For Hospital Use Only: CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Other relevant warnings and precautions:
- Removal prior to oxytocin administration
- Removal if uterine hyperstimulation is encountered or if labour commences; prior to amniotomy; if there is fetal distress; if there is evidence of maternal or fetal adverse reactions
- Caution in patients with a history of previous uterine hypertonicity, glaucoma, or childhood asthma
- Caution in patients at risk for developing disseminated intravascular coagulation
- Evaluation of cephalopelvic relationships
- Caution in patients with severe renal disease and/or severe hepatic disease accompanied by metabolic aberrations
- Not indicated for use during early or other phases of pregnancy or during lactation
- Monitoring: After insertion, the patient should remain supine and monitored for 2 hours for any evidence of uterine hyperstimulation, change in fetal heart rate or maternal blood pressure or heart rate
For more information:
Please consult product monograph https://www.ferring.ca/media/1026/cervidil-approved-pm-english-september-2006.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
RESOURCE CENTRE
HELPFUL LINKS
Use these links to access professional and patient educational resources & tools.
| Alberta Health Services | https://www.albertahealthservices.ca/main/search/Pages/Search.aspx?k=Induction+of+labour+policy |
| BC Perinatal Services | http://www.perinatalservicesbc.ca/Documents/Guidelines-Standards/Maternal/CervicalRipeningInductionLabourGuideline.pdf |
| Canadian Association of Perinatal and Women’s Health Nurses | www.capwhn.ca |
| Canadian Institute of Health Information (CIHI) Giving Birth in Canada | https://www.cihi.ca/en |
| Precare Gynecological Surgery Guide | https://precare.ca/gynonc/ |
| Society of Obstetricians and Gynecologists of Canada | www.sogc.org |
| TVASurg Gynecological Surgery videos | https://pie.med.utoronto.ca/TVASurg/all-categories/obstetrics/ |
CERVIDIL SAFETY INFORMATION
Indication and clinical use:
CERVIDIL (dinoprostone) is indicated for: Initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetrical indication for the induction of labour. CERVIDIL is not recommended in the geriatric and pediatric populations.
Contraindications:
- Patients in whom there is clinical suspicion or definite evidence of fetal distress where delivery is not imminent
- Patients with placenta previa or unexplained vaginal bleeding during this pregnancy
- Patients in whom there is evidence or strong suspicion of marked cephalopelvic disproportion
- Patients in whom oxytocic drugs are contraindicated or when prolonged contraction of the uterus may be detrimental to fetal safety or uterine integrity (previous caesarean section or major uterine surgery)
- Multipara with 6 or more previous term pregnancies
- Patients with a history of difficult labour and/or traumatic delivery
- Patients with overdistension of uterus (multiple pregnancy, polyhydramnios)
- Patients with fetal malpresentation
- Patients with a history of epilepsy whose seizures are poorly controlled
- Should not be used simultaneously with other oxytocics
- Should not be used when there is a history of, or current pelvic inflammatory disease, unless adequate prior treatment has been instituted
Most serious warnings and precautions:
For Hospital Use Only: CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Other relevant warnings and precautions:
- Removal prior to oxytocin administration
- Removal if uterine hyperstimulation is encountered or if labour commences; prior to amniotomy; if there is fetal distress; if there is evidence of maternal or fetal adverse reactions
- Caution in patients with a history of previous uterine hypertonicity, glaucoma, or childhood asthma
- Caution in patients at risk for developing disseminated intravascular coagulation
- Evaluation of cephalopelvic relationships
- Caution in patients with severe renal disease and/or severe hepatic disease accompanied by metabolic aberrations
- Not indicated for use during early or other phases of pregnancy or during lactation
- Monitoring: After insertion, the patient should remain supine and monitored for 2 hours for any evidence of uterine hyperstimulation, change in fetal heart rate or maternal blood pressure or heart rate
For more information:
Please consult product monograph https://www.ferring.ca/media/1026/cervidil-approved-pm-english-september-2006.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
References:
- CERVIDIL® (dinoprostone) Product Monograph. Ferring Inc. September 29, 2006.
- Rayburn WF et al. An intravaginal controlled-release prostaglandin E2 pessary for cervical ripening and initiation of labor at term. Obstet Gynecol 1992;79:374-379.
- SOGC Labour Guidelines https://www.jogc.com/article/S1701-2163(15)30842-2/pdf
EMAIL: CA0-MEDICALINFORMATI@FERRING.COM .PHONE: 1-866-384-1314
© 2020 FERRING INC.
All rights reserved.
Cervidil® and Duratocin® are registered trademarks of Ferring B.V.


 PODCASTS
PODCASTS