THE CHAMPION STUDY
CARBETOCIN HAEMORRHAGE PREVENTION2
A COLLABORATION OF THE WHO, FERRING AND MERCK FOR MOTHERS
- Ferring Pharmaceuticals and Merck for Mothers, MSD’s charitable initiative, approached the World Health Organization (WHO) in order to explore heat-stable carbetocin2,3
- The CHAMPION trial, studied a heat-stable formulation of carbetocin vs. oxytocin and demonstrated that the heat-stable formulation of carbetocin was non-inferior to oxytocin in the proportion of women with blood loss ≥500 mL or the use of additional uterotonic agents; 14.5% carbetocin vs 14.4% oxytocin [relative risk,1.02; 95% CI, 0.95 to 1.06]
— The proportion of women with blood loss of at least 1000 mL at 1 hour,and up to 2 hours for women who continued to bleed after 1 hour was 1.51% in the carbetocin group and 1.45% in the oxytocin group (relative risk, 1.04;95% CI, 0.87 to 1.25), with the confidence interval crossing the margin of noninferiority*
-
* Randomized, double-blind, noninferiority trial. 23 study sites, 10 countries. n= 29,645 women. Intramuscular injections of heat-stable carbetocin (at a dose of 100 mcg) was compared with oxytocin (at a dose of 10 IU) administered immediately after vaginal birth. Primary outcomes: Proportion of women with blood loss of >500 mL or the use of additional uterotonic agents and proportion of women with blood loss of at least 1000 mL, and up to 2 hours for women who continued to bleed after 1 hour. Noninferiority margins for the relative risks of these outcomes were 1.16 and 1.23, respectively.
ACTION AND CLINICAL PHARMACOLOGY
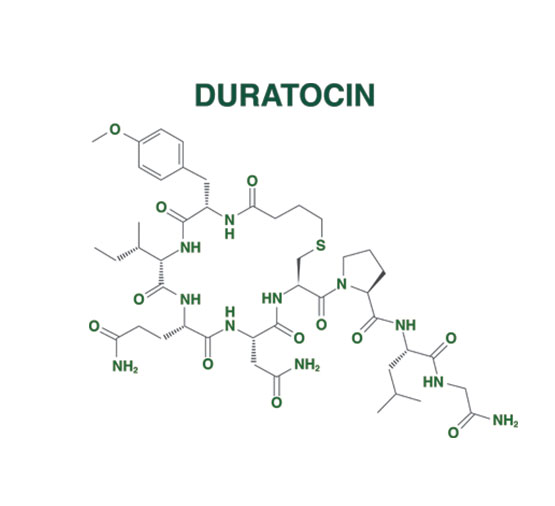
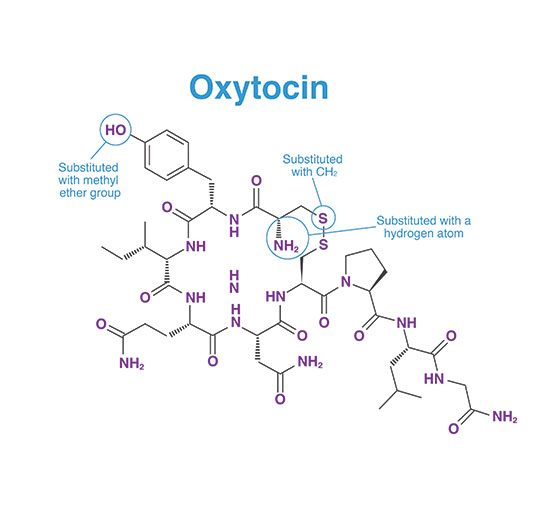
DURATOCIN STRUCTURE1*
- DURATOCIN is an analogue of oxytocin
CARBETOCIN WAS CREATED BY MAKING SEVERAL MODIFICATIONS TO THE OXYTOCIN STRUCTURE4
PHARMACOLOGICAL ACTION OF CARBETOCIN1*
- DURATOCIN selectively binds to oxytocin receptors on the smooth musculature of the uterus
- Binding results in rhythmic contractions of the uterus, increased frequency of existing contractions, and increased uterine tone
ONSET OF ACTION1*
- A firm uterine contraction is obtained within 2 minutes, with either intravenous or intramuscular route
DURATION OF ACTION1*
- The duration of action of a single intravenous injection of carbetocin on uterine activity is approximately 1 hour
ELIMINATION HALF-LIFE1*
- Based on healthy female subjects in the dose range of 400 to 800 mcg
- Recommended DURATOCIN dose is 100 mcg
- DURATOCIN shows a biphasic elimination after intravenous administration with linear pharmacokinetics in the dose range of 400 to 800 mcg.
- Half-life is 33 minutes after IV administration
- Half-life is 55 minutes after IM administration – peak concentrations are reached after 30 minutes
-
* Clinical significance is unknown.
SAFETY PROFILE OVERVIEW1
VAGINAL DELIVERY*
- In a large, randomized, active controlled, double-blind clinical trial, 14,754 subjects received carbetocin (IM) and 14,743 subjects received oxytocin (IM). Carbetocin (100 mcg IM) and oxytocin (10 IU IM) have a similar safety profile in the prevention of PPH following vaginal delivery. Table 1 below summarizes the TEAEs.1
- Most frequent TEAEs for carbetocin (≥0.2% and <1%) were anemia, abdominal pain, vomiting, pyrexia, post-procedural swelling and postpartum haemorrhage
TABLE 1: SUMMARY OF TREATMENT-EMERGENT ADVERSE EVENTS (TEAEs) IN STUDY A65870
| CARBETOCIN N=14,754 |
OXYTOCIN N=14,743 |
TOTAL N=29,497 |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Participants with at least one TEAE | 595 | 4.03 | 578 | 3.92 | 595 | 3.98 |
CAESAREAN SECTION DELIVERY
- The adverse drug reactions observed with carbetocin during the clinical trials were of the same type and frequency as the adverse events observed with oxytocin and placebo when administered after caesarean section under epidural or spinal anesthesia.
- Very common ADRs in clinical trials of elective caesarean section (>1/10): Headache, tremor, hypotension, flushing, nausea, abdominal pain, vomiting, pruritus, feeling of warmth
-
* A randomized, active controlled, double-blind, parallel group trial was conducted in healthy pregnant women undergoing vaginal delivery to establish the efficacy of carbetocin (IM) in the prevention of postpartum haemorrhage following vaginal delivery. Patients were randomized to receive a single IM of either DURATOCIN 100 mcg IM (n= 14,771) or oxytocin 10 IU IM (n=14,768). Primary endpoint: blood loss of ≥500 mL or use of additional uterotonics
DURATOCIN EMERGENCY CAESAREAN EFFICACY
DURATOCIN IV VS. OXYTOCIN IV
Ferring DURATOCIN (100 mcg IV+Ringer’s lactate solution 10 mL injected directly into the vein over 2 min) showed significantly better outcomes vs. emergency caesarean with oxytocin (20 IU diluted in 1000 mL of Ringer’s lactate solution, administered intravenously at a rate of 125 mL/hr)
IN PREGNANT WOMEN UNDERGOING EMERGENCY CAESAREAN
- 2% vs. 13% oxytocin in postpartum hemorrhage (p=0.03)
- Estimated blood loss 689 ± 580 mL vs. 1027 ± 659 mL oxytocin (p=0.002)
- 0 vs. 16% oxytocin in need for transfusion (p=0.04)
- 2% vs. 71% oxytocin in patients who needed additional uterotonics (p=0.002)
-
‡ A randomised, active-controlled, double-blind, parallel-group trial compared the effectiveness of DURATOCIN 100 mcg IV (n=188) and oxytocin 5 IU IV< (n= 189) in elective and emergency caesarean section, administered after caesarean section for prevention of postpartum haemorrhage (PPH). Primary endpoint: Incidence of need for additional oxytocin intervention1
DURATOCIN FOR PREVENTION OF POSTPARTUM HAEMORRHAGE IN OBESE NULLIPAROUS WOMEN
- El-Behery et al. reported a significant difference in the amount of estimated blood loss and in the incidence of primary postpartum haemorrhage
(≥ 1000 mL) in both groups (carbetocin 100 mcg IV; oxytocin= 20 IU, 8 hour IV infusion).5†§-
— Incidence of blood loss in the carbetocin group was 689 ± 580 mL vs. 1027 ± 659 mLs for the oxytocin group (p=0.002) — Incidence of PPH in carbetocin group was 2/90 or 2.2% vs. 12/90 or 13.33% for the oxytocin group (p=0.03)
-
- Haemoglobin levels before and 24-h postpartum were similar
- Mean hemoglobin decrease g/dL: carbetocin=1.74 vs. oxytocin=0.94 (p=0.03)
- 2.22% of patients in the carbetocin group versus 71.11% of patients in the oxytocin group needed additional uterotonics (p=0.002)
- Uterine contractility was better in the carbetocin group at 2 and 12 hours postpartum (p<0.05)
-
† The primary outcome measure was major primary postpartum hemorrhage defined as blood loss ≥1000 mL within 24 hours of delivery as per the definition of PPH by the World Health Organization § A randomized, active-controlled, double-blind, double-dummy, parallel-group trial of obese (BMI>30), nulliparous pregnant women undergoing emergency caesarean. DURATOCIN 100 mcg IV (n=90), oxytocin 20 IU 8 h IV infusion (n= 90). Primary endpoint: blood loss ≥1000 mL within 24 h of delivery. Mean hemoglobin decrease g/dL: carbetocin=1.74 vs. oxytocin= 0.94
PHARMACOKINETIC PARAMETERS OF DURATOCIN 100 MCG
PARAMETER: GEOMETRIC MEAN (GEOMETRIC %CV)
| CARBETOCIN 100 mcg IV (N = 19) |
CARBETOCIN 100 mcg IM (N = 20) |
|
|---|---|---|
| AUC0-∞ (ng*h/mL) | 2.762 (21.6%) | 2.147 (18.7%) |
| AUCt (ng*h/mL) | 2.697 (21.8%) | 2.022 (20.3%) |
| Cmax (ng/mL) | 7.232 (17.4%)a | 1.030 (30.4%) |
| tmax (hour)b | NA | 0.500 (0.250, 0.750) |
| t1/2 (hour) | 0.5480 (25.8%) | 0.9157 (28.4%) |
AUC0-∞ = area under the concentration-time curve from time 0 extrapolated to infinity; AUCt = area under the concentration-time curve from time 0 to the last quantifiable concentration; Cmax = observed maximum concentration; %CV = coefficient of variation (percent); IM = intramuscular; IV = intravenous; NA = not applicable; tmax = time of observed maximum concentration, t1/2 = terminal half-life.
aMeasured concentration at 5 minutes postdose. bResults presented are median (minimum, maximum).
DURATOCIN SAFETY INFORMATION
Indication and clinical use:
DURATOCIN (carbetocin injection) is indicated for the prevention of postpartum haemorrhage by controlling uterine atony
Contraindications:
- Due to its long duration of action relative to oxytocin, uterine contractions produced by DURATOCIN cannot be stopped by simply discontinuing the medication.
DURATOCIN should not be administered:
- In pregnancy – prior to delivery of the infant for any reason, including elective or medical induction of labour
- In patients with a history of hypersensitivity to oxytocin or carbetocin
- In patients with serious cardiovascular disorders
- In children
Relevant warnings and precautions:
- Use only at well-equipped specialist obstetrics units
- Some patients may not have an adequate uterine contraction after a single injection – administration should not be repeated and more aggressive treatment with other uterotonic drugs like oxytocin or ergometrine is warranted
- In persistent bleeding, the presence of retained placental fragments, coagulopathy, or trauma to the genital tract should be ruled out
- DURATOCIN is intended for single administration only, intramuscular (IM) or intravenous (IV)
- Significant antidiuretic effect is not anticipated and has not been demonstrated at the recommended dose
- Due to antidiuretic effects, risk of water intoxication cannot be excluded
- Monitor patients with eclampsia and pre-eclampsia for all signs and symptoms
- Not studied in patients with known coagulopathy or evidence of liver, renal or endocrine disease
- Use with extreme caution in patients with cardiovascular disease, especially coronary artery disease
- Not studied in gestational diabetes mellitus
- Use cautiously in migraine and epilepsy
- Use cautiously in asthma
- Nursing women – the small amount of DURATOCIN transferred into breast milk or colostrum after a single 70 mcg dose injection would not be expected to present a significant safety concern – no sufficient evidence to determine if it stimulates milk let-down
- Not recommended for Pediatrics (< 18 years) or Geriatrics (> 65 years)
For more information:
Please consult product monograph at https://www.ferring.ca/media/1237/duratocin-vial-pm-eng-21feb2020.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
DURATOCIN ADMINISTRATION
- Room temperature stable DURATOCIN can be administered in vaginal (IM or IV) or caesarean section (IV) deliveries1
IM OR IV ADMINISTRATION IN VAGINAL DELIVERY1
- A single dose of 100 mcg (1 mL) of DURATOCIN is given by intramuscular injection or slowly over 1 minute by intravenous bolus injection, under adequate medical supervision
- DURATOCIN must be given as soon as possible after delivery of the infant, preferably before the delivery of the placenta
- No further doses of DURATOCIN should be given
IV ADMINISTRATION IN CAESAREAN SECTION1
- A single intravenous dose of 100 mcg (1 mL) of DURATOCIN is given by bolus injection, slowly over 1 minute, only when delivery of the infant has been completed by caesarean section under epidural or spinal anaesthesia
- DURATOCIN can be given before or after delivery of the placenta
- No further doses of DURATOCIN should be given
DURATOCIN IS NOW AVAILABLE IN ROOM TEMPERATURE STABLE VIALS
DURATOCIN SAFETY INFORMATION
Indication and clinical use:
DURATOCIN (carbetocin injection) is indicated for the prevention of postpartum haemorrhage by controlling uterine atony
Contraindications:
- Due to its long duration of action relative to oxytocin, uterine contractions produced
by DURATOCIN cannot be stopped by simply discontinuing the medication.
DURATOCIN should not be administered:
- In pregnancy – prior to delivery of the infant for any reason, including elective or medical induction of labour
- In patients with a history of hypersensitivity to oxytocin or carbetocin
- In patients with serious cardiovascular disorders
- In children
Relevant warnings and precautions:
- Use only at well-equipped specialist obstetrics units
- Some patients may not have an adequate uterine contraction after a single injection – administration should not be repeated and more aggressive treatment with other uterotonic drugs like oxytocin or ergometrine is warranted
- In persistent bleeding, the presence of retained placental fragments, coagulopathy, or trauma to the genital tract should be ruled out
- DURATOCIN is intended for single administration only, intramuscular (IM) or intravenous (IV)
- Significant antidiuretic effect is not anticipated and has not been demonstrated at the recommended dose
- Due to antidiuretic effects, risk of water intoxication cannot be excluded
- Monitor patients with eclampsia and pre-eclampsia for all signs and symptoms
- Not studied in patients with known coagulopathy or evidence of liver, renal or endocrine disease
- Use with extreme caution in patients with cardiovascular disease, especially coronary artery disease
- Not studied in gestational diabetes mellitus
- Use cautiously in migraine and epilepsy
- Use cautiously in asthma
- Nursing women – the small amount of DURATOCIN transferred into breast milk or colostrum after a single 70 mcg dose injection would not be expected to present a significant safety concern – no sufficient evidence to determine if it stimulates milk let-down
- Not recommended for Pediatrics (< 18 years) or Geriatrics (> 65 years)
For more information:
Please consult product monograph at https://www.ferring.ca/media/1237/duratocin-vial-pm-eng-21feb2020.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
DURATOCIN KEY FEATURES OVERVIEW
- Single 100 mcg dose for vaginal (IM or IV) or caesarean delivery (IV)1
- Single IM or IV 100 mcg dose is administered by bolus injection, slowly over 1 minute for vaginal delivery
- Shown to reduce the need for additional uterotonic administration in pregnant women with an increased risk of postpartum haemorrhage undergoing C-section1
– 2% of carbetocin subjects needed additional uterotonics vs. 71% of oxytocin subjects (p=0.0002)‡
-
‡ A randomized, active-controlled, double-blind, parallel-group trial compared the effectiveness of DURATOCIN 100 mcg IV (n=188) and oxytocin 5 IU IV (n=189) in elective and emergency caesarean section, administered after caesarean section for prevention of postpartum haemorrhage (PPH). Primary endpoint: Incidence of need for additional oxytocin intervention1
DEMONSTRATED EFFICACY IN PREVENTING POSTPARTUM HEMORRHAGE BY CONTROLLING UTERINE ATONY1
- DURATOCIN IM in vaginal deliveries was shown to be at least as effective as oxytocin IM in blood loss of at least 500 mL or the need for additional uterotonic agents1*
- Uterine contractility was shown to be superior with DURATOCIN IV vs. oxytocin at 2 hours and 12 hours after emergency caesarean section (p<0.05)1*
-
* Double-blind, randomized-controlled trial. n=180 pregnant women with BMI>30 randomized to receive oxytocin (2 ampoules; 20 IU) or DURATOCIN (100 mcg) by IV, during caesarean surgery. Primary outcome: major primary PPH ≥1000 mL within 24 hours of delivery. Secondary outcomes: hemoglobin and hematocrit changes pre and post-delivery, use of further ecobolics, uterine tone and adverse effects. Study results: Major primary PPH: DURATOCIN = 2.22% vs. oxytocin = 13.33%; p=0.03. Postpartum hemoglobin level: DURATOCIN = 11.4 ± 1.76 g/dL vs. oxytocin = 10.8 ± 1.68 g/dL; p=0.09. Soft uterine tone: DURATOCIN = 2.22% vs. oxytocin=15.55%; p<0.05. Need for additional uterotonics: DURATOCIN = 2.22% vs. oxytocin= 71.11%; p<0.005
DURATOCIN SAFETY INFORMATION
Indication and clinical use:
DURATOCIN (carbetocin injection) is indicated for the prevention of postpartum haemorrhage by controlling uterine atony
Contraindications:
- Due to its long duration of action relative to oxytocin, uterine contractions produced by DURATOCIN cannot be stopped by simply discontinuing the medication.
DURATOCIN should not be administered:
- In pregnancy – prior to delivery of the infant for any reason, including elective or medical induction of labour
- In patients with a history of hypersensitivity to oxytocin or carbetocin
- In patients with serious cardiovascular disorders
- In children
Relevant warnings and precautions:
- Use only at well-equipped specialist obstetrics units
- Some patients may not have an adequate uterine contraction after a single injection – administration should not be repeated and more aggressive treatment with other uterotonic drugs like oxytocin or ergometrine is warranted
- In persistent bleeding, the presence of retained placental fragments, coagulopathy, or trauma to the genital tract should be ruled out
- DURATOCIN is intended for single administration only, intramuscular (IM) or intravenous (IV)
- Significant antidiuretic effect is not anticipated and has not been demonstrated at the recommended dose
- Due to antidiuretic effects, risk of water intoxication cannot be excluded
- Monitor patients with eclampsia and pre-eclampsia for all signs and symptoms
- Not studied in patients with known coagulopathy or evidence of liver, renal or endocrine disease
- Use with extreme caution in patients with cardiovascular disease, especially coronary artery disease
- Not studied in gestational diabetes mellitus
- Use cautiously in migraine and epilepsy
- Use cautiously in asthma
- Nursing women – the small amount of DURATOCIN transferred into breast milk or colostrum after a single 70 mcg dose injection would not be expected to present a significant safety concern – no sufficient evidence to determine if it stimulates milk let-down
- Not recommended for Pediatrics (< 18 years) or Geriatrics (> 65 years)
For more information:
Please consult product monograph at https://www.ferring.ca/media/1237/duratocin-vial-pm-eng-21feb2020.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
HEALTH CARE PROFESSIONAL SUPPORT RESOURCES
DURATOCIN SAFETY INFORMATION
Indication and clinical use:
DURATOCIN (carbetocin injection) is indicated for the prevention of postpartum haemorrhage by controlling uterine atony
Contraindications:
- Due to its long duration of action relative to oxytocin, uterine contractions produced by DURATOCIN cannot be stopped by simply discontinuing the medication.
DURATOCIN should not be administered:
- In pregnancy – prior to delivery of the infant for any reason, including elective or medical induction of labour
- In patients with a history of hypersensitivity to oxytocin or carbetocin
- In patients with serious cardiovascular disorders
- In children
Relevant warnings and precautions:
- Use only at well-equipped specialist obstetrics units
- Some patients may not have an adequate uterine contraction after a single injection – administration should not be repeated and more aggressive treatment with other uterotonic drugs like oxytocin or ergometrine is warranted
- In persistent bleeding, the presence of retained placental fragments, coagulopathy, or trauma to the genital tract should be ruled out
- DURATOCIN is intended for single administration only, intramuscular (IM) or intravenous (IV)
- Significant antidiuretic effect is not anticipated and has not been demonstrated at the recommended dose
- Due to antidiuretic effects, risk of water intoxication cannot be excluded
- Monitor patients with eclampsia and pre-eclampsia for all signs and symptoms
- Not studied in patients with known coagulopathy or evidence of liver, renal or endocrine disease
- Use with extreme caution in patients with cardiovascular disease, especially coronary artery disease
- Not studied in gestational diabetes mellitus
- Use cautiously in migraine and epilepsy
- Use cautiously in asthma
- Nursing women – the small amount of DURATOCIN transferred into breast milk or colostrum after a single 70 mcg dose injection would not be expected to present a significant safety concern – no sufficient evidence to determine if it stimulates milk let-down
- Not recommended for Pediatrics (< 18 years) or Geriatrics (> 65 years)
For more information:
Please consult product monograph at https://www.ferring.ca/media/1237/duratocin-vial-pm-eng-21feb2020.pdf for important information relating to adverse reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-866-384-1314.
References:
- PrDURATOCIN® Product Monograph. Ferring Inc. February 21, 2020.
- Widmer M, et al. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med 2018;379(8):743e52. (CHAMPION Study) https://www.nejm.org/doi/full/10.1056/NEJMoa1805489
- WHO recommendations: Uterotonics for the prevention of postpartum haemorrhage. Web annex 2: Carbetocin versus placebo or no treatment: Evidence to Decision framework. Geneva: World Health Organization; 2018 (WHO/RHR/18.29). Licence: CC BY-NC-SA 3.0 IGO.
- Cordovani D et al. Carbetocin at elective Caesarean delivery: a randomized controlled trial to determine the effective dose. Can J Anesth/J Can Anesth. 2012:59:751–757. DOI 10.1007/s12630-012-9728-2
- El Behery, et al. Carbetocin versus oxytocin for prevention of postpartum haemorrhage in obese nulliparous women undergoing emergency casesarean delivery. J Matern Fetal Neonatal Med 2016;29(8):1257-60. DOI: 10.3109/14767058.2015.1043882
® Registered trademark of Ferring B.V.
EMAIL: CA0-MEDICALINFORMATI@FERRING.COM .PHONE: 1-866-384-1314
© 2021 FERRING INC.
All rights reserved.
Cervidil® and Duratocin® are registered trademarks of Ferring B.V.


 PODCASTS
PODCASTS